Non-denatured bone nutrition-bone health series
I. Overview
Our Non-Denatured Nutritional Products for bone health include: Whole Bone Nutrition Powder, Non-Denatured Bone Nutrition Ultra Micro Powder, Non-Denatured Bone Protein Calcium Ultra Micro Powder, and Non-Denatured Type I Collagen Ultra Micro Powder. These products are made from fresh, age-appropriate cow femur (including joints) as the primary raw material, which ensures the highest quality collagen, glycosaminoglycans, and bone proteins. The manufacturing process involves super-low temperature ultra-microization and airflow principle-based super-low temperature separation technology, ensuring the preservation of the non-denatured structure. This preserves the functional properties of the ingredients, making them ideal for supporting bone health and tissue repair.
The unique non-denatured nature of these products ensures that they retain their natural active structure, which is essential for the body to fully utilize the bioactive compounds. This innovative technology allows for superior absorption and efficacy in supporting bone and joint health.
II. Component Ratios

*Collagen primarily consists of Type I collagen (over 90%), with a small amount of Type II collagen (less than 10%)
III. Natural Active Structure
3.1 Primary Structure
3.1.1 Type I Collagen: Dominated by Gly-Pro-X or Gly-X-Hyp repeating amino acid sequences (peptide bonds)
3.1.2 Glycosaminoglycans
Hyaluronic Acid (HA): A simple repeating linear polysaccharide chain formed by alternating β(1→3) and β(1→4) glycosidic bonds between D-glucuronic acid and N-acetylglucosamine.
Chondroitin sulfate (CS): A series of microscopically heterogeneous linear polysaccharide chains with a basic backbone of alternately linked D-glucuronic acid and N-acetylgalactosamine, featuring complex and variable sulfation modifications at specific positions on N-acetylgalactosamine (and occasionally glucuronic acid).
3.2 Higher Structures
3.2.1 Type I Collagen
Secondary Structure: α-peptide chain left-handed helical structure (primarily hydrogen bonds)
Tertiary Structure: Triple helix structure (peptide bonds, hydrogen bonds, van der Waals forces, hydrophobic interactions, aldol condensation covalent cross-links)
3.2.2 Higher Structures of Glycosaminoglycans (GAGs)
Hyaluronic acid higher structure: Forms a randomly coiled, highly hydrated network in solution.
Chondroitin sulfate higher structure: Covalently bound to core proteins to form proteoglycans.
3.3 Superstructure (Supramolecular Covalent Cross-linking)
Between Type I collagen macromolecules: Pyridinoline cross-linking structure.
Between osteocalcin and hydroxyapatite (HAP): Calcium chelated by γ-carboxyglutamic acid.
IV. Mechanism of Action and Effects
Whole Bone Nutrition Powder, Non-denatured Bone Nutrition Ultrafine Powder, Non-denatured Bone Protein Calcium Ultrafine Powder, and Non-denatured Type I Collagen Ultrafine Powder not only provide comprehensive, balanced nutrients essential for bone tissue growth and repair but also deliver the benefits of “active structures.”
4.1 Mechanism and Effects of Calcium Absorption Enhancement
4.1.1 Mechanism: The supercovalent structure (γ-carboxyglutamic acid chelated calcium) between osteocalcin in non-denatured bone protein calcium ultrafine powder and hydroxyapatite (HAP) is protected by the protein's three-dimensional structure, preventing contact with gastric acid. This allows the complex to reach the intestines intact, Under the action of intestinal digestive enzymes, it breaks down into soluble calcium molecules such as γ-carboxyglutamic acid chelated calcium or small-molecule peptide-bound calcium. This increases the concentration of calcium molecules in the intestine, enabling absorption via the paracellular pathway driven by concentration gradients.
4.1.2 Effect: This absorption relies on concentration gradients and is independent of calcium-binding protein (CaBP) saturation. For patients with reduced CaBP levels due to growth, development, or aging, leading to limited calcium absorption, this achieves physiological compensation for calcium uptake.
4.2 Mechanism and Effect of Promoting Calcium Osteogenesis
4.2.1 Mechanism: Calcium osteogenesis is regulated by multiple factors in both the blood and bone tissue environments.
The blood environment is primarily influenced by the calcium-phosphorus concentration product coefficient. Non-denatured bone protein calcium ultrafine powder is gradually digested and decomposed in the intestine into soluble calcium molecules such as γ-carboxyglutamic acid chelated calcium or small-molecule peptide-bound calcium, allowing for slow absorption. Its calcium-to-phosphorus ratio approaches 2:1, maximizing the calcium-phosphorus concentration product and promoting calcium flow from blood to bone tissue.
The bone tissue environment is influenced by three factors: the HAP nano-region of type I collagen, carboxylation of glutamic acid residues in osteocalcin, and regulation by bridging proteins and bone adhesion molecules.
The triple-helix structure of the non-denatured type I collagen fragments it contains can activate the intestinal lymphatic system, regulating immunity, suppressing inflammatory responses, and slowing Type I collagen degradation. Its degradation products—small molecules like cross-linked peptides and conjugated peptides—induce Type I collagen structure formation through multiple mechanisms (substrate, molecular chaperone, signaling). Further broken down into amino acid monomers, they directly participate in collagen synthesis, providing ample nano-regions for HAP and maximizing collagen-HAP nano-regions.
Its non-denatured bone proteins bind calcium to form calcium-bound γ-carboxyglutamic acid or calcium-bound small peptides. Alternatively, they activate γ-carboxyglutamyl carboxylase as substrates or through signal transduction, catalyzing the carboxylation of three glutamic acid residues on osteocalcin. This provides calcium-binding sites, maximizing calcium attachment points.
The non-denatured desmin and non-denatured osteonectin it contains can promote the synthesis of corresponding proteins, spatially confine HAP within type I collagen, anchor collagen to HAP, and prevent bone calcium loss.
4.2.2 Effect: Calcium can enter, be stored, and remain.
4.3 Mechanism and Effects of Increasing Bone Protein Content
Bone proteins include collagen (primarily type I collagen) and glycoproteins (mainly osteocalcin, fibronectin, osteonectin, and over 10 others). The mechanism for increasing their content is fundamentally similar, exemplified by non-denatured type I collagen ultrafine powder.
4.3.1 Mechanism
Intestinal Immune Regulation Mechanism: Non-denatured type I collagen ultrafine powder possesses a three-dimensional structure that resists degradation by gastric juices, enabling direct physical contact with the intestinal lymphatic system. This activates immature T cells to differentiate into specific Tregs, suppressing the immune system's erroneous attack on type I collagen. It promotes the secretion of anti-inflammatory factors to inhibit chronic inflammation and slow the degradation of type I collagen.
Intracellular Enzyme Activity Regulation Mechanism: Non-denatured Type I collagen ultrafine powder contains intact primary, high, and super covalent cross-linking structures. Intestinal digestive enzymes selectively break down primary (peptide bond) covalent cross-links while preserving high and super covalent cross-links. This process releases small molecules such as cross-linked peptides and conjugated peptides, or serve as substrates, molecular chaperones, or signaling molecules to induce type I collagen structural formation.
Nutrient provision: Cross-linked peptides, conjugated peptides, and other small molecules are further broken down into individual amino acids, directly participating in collagen synthesis.
4.3.2 Effects
This dual-action regulation—inhibiting degradation while promoting synthesis—yields significantly greater efficacy than the nutrient-supplying role of denatured proteins or peptides.
4.4 Mechanism and Effects of Enhancing Glycosaminoglycans (GAGs)
4.4.1 Mechanism
Non-denatured glycosaminoglycan ultrafine particles deliver fragments of HA, CS, and KS. These are readily digested into low-molecular-weight fragments, oligosaccharides, and disaccharide units. They activate chondrocytes, inhibit matrix-degrading enzymes (MMPs or ADAMTS), regulate immunity, and exert anti-inflammatory and analgesic effects. Intracellularly, they further break down into individual glycosaminoglycan molecules, directly participating in HA, CS, and KS synthesis.
4.4.2 Effects
Increases GAGs content.
V. Tissue-Derived
5.1 Tissue-Derived Determined by Structure
The primary functions of non-denatured nutrition (immunomodulation, enzyme activity regulation) are achieved through its natural active structure. This structure exhibits high specificity, with structure determining tissue-derived properties.
5.2 Tissue-Derived Nature of Non-Denatured Bone Nutrition Series
Whole Bone Nutrition Powder, Non-Denatured Bone Nutrition Ultrafine Powder, Non-Denatured Bone Protein Calcium Ultrafine Powder, and Non-Denatured Type I Collagen Ultrafine Powder all utilize fresh, age-appropriate bovine shank bones (including joints) as raw materials. Their primary protein is Type I collagen, and their tissue-derived nature should be determined based on Type I collagen. Although skin tissue Type I collagen shares genetic homology with bone tissue and identical amino acid sequences, differences in microenvironmental modifications result in distinct higher-order and ultrastructural arrangements. Therefore, skin tissue is not considered homologous to the non-denatured bone nutrition series. Primary homologous tissues include: hard bone tissue, intervertebral discs, menisci, and minor amounts of articular cartilage.
VI. Suitable Population
6.1 Nutritional Needs: Suitable for all individuals with calcium deficiency, particularly infants, young children, and the elderly.
6.2 Bone Growth Needs: Suitable for those requiring height increase during developmental stages and those seeking to slow height loss during aging.
6.2 Bone Mass Enhancement Needs
6.2.1 Individuals with rickets symptoms during growth and development: Such as delayed fontanelle closure, square fontanelle, flat head, pigeon chest, funnel chest, beaded changes, bowlegs or knock knees, scoliosis, etc.
6.2.2 Middle-aged and elderly individuals: Those exhibiting signs of bone hyperplasia, height loss, kyphosis, osteoporosis, herniated discs, etc.
6.3 Joint Degeneration Repair Needs: Patients with degenerative osteoarthritis, especially those with meniscus injuries or inflammation.
6.4 Bone Injury Healing Needs: Bone injury healing or bone cement implantation growth, etc.
Non-Degenerative Joint Nutrition - Joint Health Series
I. Overview
The Joint Health Series non-denatured nutritional products include: Total Joint Nutrition Powder, Non-Denatured Joint Nutrition Ultrafine Powder, Non-Denatured Cartilage Nutrition Ultrafine Powder, and Non-Denatured Type II Collagen Ultrafine Powder. All are functional food ingredients produced from fresh, age-appropriate bovine knee joints or cartilage. Manufactured according to relevant standards using ultra-low-temperature ultrafine processing (patented national invention technology) and wind cabinet principle ultra-low-temperature separation technology (proprietary technology).
II. Component Ratios

*Collagen primarily consists of Type II collagen (over 90%), with a small amount of Type I collagen (less than 10%).
III. Natural Active Structure
3.1 Primary Structure
3.1.1 Type II Collagen: Primarily composed of Gly-Pro-X or Gly-X-Hyp repeating amino acid sequences (peptide bond)
3.1.2 Glycosaminoglycans
Hyaluronic acid (HA): A simple repeating linear polysaccharide chain formed by alternating β(1→3) and β(1→4) glycosidic bonds between D-glucuronic acid and N-acetylglucosamine.
Chondroitin sulfate (CS): A series of microscopically heterogeneous linear polysaccharide chains formed by alternating D-glucuronic acid and N-acetylgalactosamine units as the basic backbone, with complex and variable sulfation modifications occurring at specific positions on N-acetylgalactosamine (and occasionally glucuronic acid) units.
3.2 Higher Structures
3.2.1 Type II Collagen
Secondary Structure: α-peptide chain left-handed helical structure (primarily hydrogen bonds)
Tertiary Structure: Triple helix structure (peptide bonds, hydrogen bonds, van der Waals forces, hydrophobic interactions, aldol condensation covalent cross-links)
3.2.2 Glycosaminoglycans (GAGs) Higher-Order Structure
Hyaluronic Acid Higher-Order Structure: Forms a randomly coiled, highly hydrated network structure in solution.
Chondroitin Sulfate Higher-Order Structure: Covalently bound to core proteins to form proteoglycans.
3.3 Supramolecular Structure (Supramolecular Covalent Cross-Linking)
Type II Collagen Intermolecular Cross-Linking: Hydroxypyridoline.
Osteocalcin and hydroxyapatite (HAP): γ-carboxyglutamic acid chelates calcium.
IV. Mechanism of Action and Effects
Whole Joint Nutrition Powder, Non-denatured Joint Nutrition Ultrafine Powder, Non-denatured Cartilage Nutrition Ultrafine Powder, and Non-denatured Type II Collagen Ultrafine Powder not only provide comprehensive, balanced nutrients for joint tissues but also exert “active structural” effects.
4.1 Mechanism and Effects of Calcium Absorption Promotion
4.1.1 Mechanism: The super-covalent structure (γ-carboxyglutamic acid chelated calcium) between osteocalcin and hydroxyapatite (HAP) in non-denatured bone protein calcium ultrafine powder avoids contact with gastric juices under the protection of the protein's three-dimensional structure. This allows it to reach the intestines intact, where it is broken down by intestinal digestive enzymes into soluble calcium molecules such as γ -carboxyglutamic acid chelated calcium or small-molecule peptide-bound calcium, thereby increasing intestinal calcium concentration. Under concentration gradient effects, calcium is absorbed via the paracellular pathway.
4.1.2 Effect: This absorption relies on concentration gradients and is independent of calcium-binding protein (CaBP) saturation. For patients with reduced CaBP levels due to growth, development, or aging, leading to limited calcium absorption, this achieves physiological compensation for calcium uptake.
4.2 Mechanism and Effect of Promoting Calcium Osteogenesis
4.2.1 Mechanism: Calcium osteogenesis is regulated by multiple factors in both the blood and bone tissue environments.
The blood environment is primarily influenced by the calcium-phosphorus concentration product coefficient. Non-denatured bone protein calcium ultrafine powder is gradually digested and broken down in the intestine into soluble calcium molecules such as γ-carboxyglutamic acid chelated calcium or small-molecule peptide-bound calcium, allowing for slow absorption. Its calcium-to-phosphorus ratio approaches 2:1, maximizing the calcium-phosphorus concentration product and promoting calcium flow from the blood to bone tissue.
The bone tissue environment is influenced by three factors: the HAP nanoregion of type I collagen, carboxylation of glutamic acid residues in osteocalcin, and regulation by bridgin and osteonectin.
The non-denatured type I collagen fragments within it activate the intestinal lymphatic system, modulating immunity, suppressing inflammatory responses, and slowing type I collagen degradation. Its degradation products—small molecules like cross-linked peptides and conjugated peptides—function as substrates, molecular chaperones, signaling pathways to induce type I collagen structural formation. Further degradation into amino acid monomers directly participates in collagen synthesis, providing ample nano-regions for HAP and maximizing collagen HAP nano-regions.
Its non-denatured bone proteins bind calcium to form calcium-bound γ-carboxyglutamic acid or calcium-conjugated small peptides. Alternatively, they activate γ-carboxyglutamic acid carboxylase as substrates or through signal transduction, catalyzing the carboxylation of three glutamic acid residues on osteocalcin. This provides calcium-binding sites, maximizing calcium attachment points.
The non-denatured desmin and non-denatured osteonectin it contains can promote the synthesis of corresponding proteins, spatially confine HAP within type I collagen, anchor collagen to HAP, and prevent bone calcium loss.
4.2.2 Effect: Calcium enters, is retained, and remains.
4.3 Cartilage Tissue Repair Mechanism and Effects
Articular cartilage is hyaline cartilage, with type II collagen as its primary protein.
4.3.1 Mechanism
Intestinal Immune Regulation Mechanism: Non-denatured type II collagen ultrafine powder possesses a three-dimensional structure that resists degradation of its natural active conformation by gastric juices. It reaches the intestine and physically interacts specifically with the intestinal lymphatic system, activating naive T cells to transform into specific Tregs. Tregs modulate effector T cells to prevent erroneous immune attacks on type II collagen, secrete anti-inflammatory factors to suppress chronic inflammation, and slow type II collagen degradation.
Intra-Organ Enzyme Activity Regulation Mechanism: Non-denatured type II collagen ultrafine powder contains intact primary, high-order, and super-order covalent cross-linking structures. Intestinal digestive enzymes selectively degrade primary (peptide bond) covalent cross-links while preserving high-order and super-order structures. This yields small molecules like cross-linked peptides and conjugated peptides, or serve as substrates, molecular chaperones, or signaling molecules to induce type II collagen structure formation.
Nutrient action: Small molecules like cross-linked peptides and conjugated peptides are further broken down into amino acids that participate in type II collagen synthesis.
4.3.2 Effects
This dual-action regulation—inhibiting degradation while promoting synthesis—yields significantly greater efficacy than effects mediated by denatured proteins or peptides.
4.4 Glycosaminoglycans (GAGs) Mechanism and Effects
4.4.1 Mechanism
Non-denatured GAG ultrafine particles provide fragments of HA, CS, and KS, which readily digest into low-molecular-weight fragments, oligosaccharides, and disaccharide units. These activate chondrocytes, inhibit matrix-degrading enzymes (MMPs or ADAMTS), regulate immunity, and exert anti-inflammatory and analgesic effects. They can also be further broken down intracellularly into individual glycosaminoglycan molecules, directly participating in the synthesis of HA, CS, and KS.
4.4.2 Effects
Increases GAG content.
V. Tissue-Derived
5.1 Tissue-Derived Determined by Structure
The primary functions of non-denatured nutrition (immunomodulation, enzyme activity regulation) are achieved through its natural active structure. This structure exhibits high specificity, with structure determining tissue-derived properties.
5.2 Tissue-Derived Nature of Non-Denatured Joint Nutrition Series
Whole Joint Nutrition Powder, Non-Denatured Joint Nutrition Ultrafine Powder, Non-Denatured Cartilage Nutrition Ultrafine Powder, and Non-Denatured Type II Collagen Ultrafine Powder all utilize fresh, age-appropriate bovine knee joints or cartilage as raw materials. Their primary protein is Type II collagen, with tissue origin derived from cartilage tissue rich in Type II collagen. Primary homologous tissues include: articular cartilage and minor amounts of hard bone.
VI. Suitable Population
6.1 Joint Injury Repair Needs
6.1.1 Degenerative Osteoarthritis Repair Needs
6.1.2 Joint Trauma Repair Needs
Non-Denatured Skin Nutrition - Skin Health Series
I. Overview
The Skin Health Series non-denatured nutritional products include: Whole Skin Nutrition Powder, Non-Denatured Skin Nutrition Ultrafine Powder, and Non-Denatured Type III Collagen Ultrafine Powder. All are produced from fresh, age-appropriate bovine or donkey hides. Manufactured according to relevant standards using ultra-low-temperature ultramicronization (patented national invention technology) and wind cabinet principle ultra-low-temperature separation technology (proprietary technology), these are non-denatured functional food ingredients.
II. Ingredient Ratios

III. Natural Active Structures
3.1 Primary Structure
3.1.1 Type I/III Collagen: Primarily Gly-Pro-X or Gly-X-Hyp repeating amino acid sequences (peptide bonds)
3.1.2 Glycosaminoglycans
Hyaluronic Acid (HA): A simple repeating linear polysaccharide chain formed by alternating β(1→3) and β(1→4) glycosidic bonds between D-glucuronic acid and N-acetylglucosamine.
Chondroitin sulfate (CS): A series of microscopically heterogeneous linear polysaccharide chains formed by alternating D-glucuronic acid and N-acetylgalactosamine units as the basic backbone, with complex and variable sulfation modifications occurring at specific positions on N-acetylgalactosamine (and occasionally glucuronic acid) units.
3.2 Higher Structures
3.2.1 Type I/III Collagen
Secondary Structure: α-peptide chain left-handed helical structure (primarily hydrogen bonds)
Tertiary Structure: Triple helix structure (peptide bonds, hydrogen bonds, van der Waals forces, hydrophobic interactions, aldol condensation covalent cross-links)
3.2.2 Higher Structures of Glycosaminoglycans (GAGs)
Hyaluronic acid higher structure: Forms a randomly coiled, highly hydrated network in solution.
Chondroitin sulfate higher structure: Core proteins covalently bind to form proteoglycans.
3.3 Superstructure (Supramolecular Covalent Cross-linking)
Type I collagen intermolecular cross-linking: Hydroxylysine pyridinoline and lysine pyridinoline.
IV. Mechanism of Action and Effects
Whole Skin Nutrition Powder, Non-denatured Skin Nutrition Ultrafine Powder, and Non-denatured Type III Collagen Ultrafine Powder not only provide comprehensive, balanced nutrients essential for skin tissue growth and repair but also deliver the effects of “active structures.”
4.1 Collagen Enhancement Mechanism and Effects
4.1.1 Mechanism
Intestinal Immune Regulation Mechanism: Non-denatured Type I/III collagen ultrafine powder possesses a three-dimensional structure that resists degradation of its natural active structure by gastric juices. It reaches the intestines and physically interacts with the intestinal lymphatic system, activating naive T cells to transform into specific Tregs (regulatory T cells). Tregs modulate effector T cells to prevent erroneous immune attacks against non-denatured Type I/III collagen, secrete anti-inflammatory factors to suppress chronic inflammation, and slowing the degradation of non-denatured I/III collagen.
Intracellular Enzyme Activity Regulation Mechanism: Non-denatured I/III collagen ultrafine powder contains intact primary, high, and super covalent cross-linking structures. Intestinal digestive enzymes can specifically select and break down primary (peptide bond) covalent cross-link structures while preserving high-order and super-order covalent cross-link structures. This process yields small molecules such as cross-linked peptides and conjugated peptides, which then induce the formation of I/III type collagen structures through multiple pathways including serving as substrates, molecular chaperones, or signaling molecules.
Nutrient Function: Small molecules like cross-linked peptides and conjugated peptides can further break down into single amino acid molecules, directly participating in collagen synthesis.
4.1.2 Effect
This dual-action regulation—inhibiting degradation while promoting synthesis—yields significantly greater efficacy than the nutrient function provided by denatured proteins or peptides.
4.2 Mechanism and Effect of Enhancing Glycosaminoglycans (GAGs)
4.2.1 Mechanism
Non-denatured glycosaminoglycan ultrafine particles provide HA and CS fragments that readily digest into low-molecular-weight fragments, oligosaccharides, and disaccharide units. These inhibit matrix-degrading enzymes (MMPs or ADAMTS), regulate immune responses for anti-inflammatory and analgesic effects, and can further break down intracellularly into individual glycosaminoglycan molecules to directly participate in HA and CS synthesis.
4.2.2 Effects
Enhances GAG content.
V. Tissue-Derived Properties
5.1 Tissue-Derived Properties Determined by Structure
The primary functions of non-denatured nutrition (immunomodulation, enzyme activity regulation) are achieved through its naturally active structure. This structure exhibits high specificity, with its composition defining tissue-derived properties.
5.2 Tissue-Derived Properties of Non-Denatured Skin Nutrition Series
Whole Skin Nutrition Powder, Non-denatured Skin Nutrition Ultrafine Powder, and Non-denatured Type III Collagen Ultrafine Powder all utilize fresh, age-appropriate donkey or cowhide as raw materials. Their primary proteins are Type I and III collagen, with their tissue origin being skin tissue rich in these collagen types. The main homologous tissue is: skin tissue.
VI. Suitable Populations
6.1 Skin Anti-Aging
6.2 Skin Damage Repair
Non-Denatured Brain Nutrition - Brain Health
I. Overview
Non-Denatured Brain Neurotrophic Ultrafine Powder is a non-denatured functional food ingredient produced from fresh bovine or porcine brain. It is manufactured according to relevant standards using ultra-low-temperature ultrafine grinding (patented national invention technology) and wind cabinet principle ultra-low-temperature separation technology (proprietary technology).
II. Component Ratios
2.1 General Classification
Water, lipids, proteins, inorganic salts, other key molecules. Inorganic salts and key molecules are present in very low concentrations.

2.2 Protein Composition
Brain proteins constitute approximately 30% of dry weight and can be classified by function:
2.2.1 Structural Proteins: Microtubule proteins, microfilaments (actin), neurofilament proteins.
2.2.2 Synapse-associated proteins: Ion channel proteins, neurotransmitter receptor proteins, vesicle-associated proteins, scaffold proteins.
2.2.3 Myelin-associated proteins: Myelin basic protein, proteolipid proteins, myelin-associated glycoproteins.
2.2.4 Metabolism and energy-related proteins: Mitochondrial proteins, hexokinase, enolase
2.2.5 Neurotrophic factors:
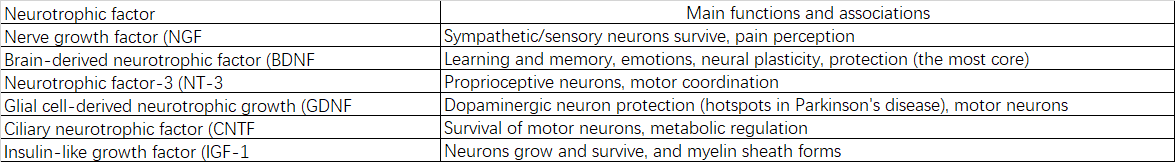
2.2.6 Signaling proteins: G proteins, protein kinases, phosphatases, etc.
2.2.7 Disease-associated proteins: β-amyloid, tau protein, α-synuclein.
2.3 Lipid composition
2.3.1 Phospholipids: Cerebroside, phosphatidylinositol, phosphatidylserine, sphingomyelin, lecithin, etc.
2.3.2 Cholesterol
2.2.3 Neutral fats
Within phospholipids and neutral fats, specific fatty acids are present: saturated fatty acids constitute approximately 30%+, while highly unsaturated fatty acids account for about 60%+ (ARA, DHA, ALA, EPA).
III. Relationship Between Brain Proteins and Aging
Brain aging is not a singular event but a systemic, progressive process involving the gradual imbalance of the brain's protein homeostasis network, the gradual depletion of functional proteins, and the gradual accumulation of abnormal proteins.
3.1 Comprehensive Decline of the Protein Homeostasis Network
All three major cellular systems maintaining protein quality control exhibit functional decline:
3.1.1 Reduced Efficiency of Protein Synthesis and Folding
Aging cells experience decreased protein synthesis rates and fidelity, increasing the likelihood of producing misfolded proteins.
The stress-induced molecular chaperone response becomes less effective, failing to properly assist newly synthesized proteins in folding correctly or repairing damaged proteins.
3.1.2 Decline in Protein Degradation System Capacity
The ubiquitin-proteasome system exhibits reduced activity, unable to promptly clear damaged or misfolded proteins; proteasome assembly is also impaired by oxidative damage, accelerating its functional decline.
The autophagy-lysosome system, particularly macroautophagy and chaperone-mediated autophagy, shows significantly reduced efficiency. This prevents effective clearance of abnormal protein aggregates and damaged organelles (e.g., mitochondria).
3.1.3 Impaired Protein Transport and Distribution
Dysfunction in structural proteins prevents the efficient delivery of synapse-required proteins and mitochondria to the distal end, while waste products cannot be promptly transported back to the cell body for processing.
3.2 Accumulation and Toxicity of Abnormal Proteins
The failure of the homeostasis network leads to the gradual accumulation of various “waste proteins” in the brain, directly damaging neurons.
3.2.1 Accumulation of Misfolded and Aggregation-Prone Proteins
Soluble oligomers of proteins such as β-amyloid, tau, and α-synuclein appear.
3.2.2 Accumulation of Abnormally Post-Translationally Modified Proteins
AGEs-modified proteins: Advanced glycation end products accumulate in the aging brain, causing protein cross-linking, stiffening, loss of function, and activation of inflammatory pathways.
Hyperphosphorylated Tau protein: Normal Tau detaches from microtubules, losing its function in stabilizing the cytoskeleton.
3.3 Loss of Key Functional Proteins
Beyond increased “waste,” “useful” proteins also diminish or fail.
3.3.1 Decreased Synapse-Associated Proteins
Downregulation of key presynaptic (e.g., synaptophysin, SNAP-25) and postsynaptic (e.g., PSD-95, NMDA/AMPA receptors) proteins directly causes synaptic structural atrophy and functional decline, forming the direct molecular basis for impaired learning and memory.
3.3.2 Decreased Neurotrophic Factors and Their Receptors
Weakened synthesis and signaling of brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and others results in insufficient supportive signals for neuronal survival, growth, and plasticity.
3.3.3 Decreased DNA Repair Proteins and Telomerase Activity
This leads to genomic instability and cellular senescence, impairing long-term neuronal survival.
3.4 Systemic Consequences
The aforementioned protein-level alterations trigger a cascade of reactions culminating in brain aging:
3.4.1 Mitochondrial Dysfunction: Damaged proteins accumulate in mitochondria, reducing ATP production and exacerbating oxidative stress.
3.4.2 Chronic Neuroinflammation: Glial cells (microglia and astrocytes) become persistently activated by abnormal proteins (e.g., Aβ oligomers), transforming from “guardians” to “destroyers” by releasing pro-inflammatory factors that damage healthy neurons.
3.4.3 Loss of Synaptic Plasticity: Imbalances in key proteins weaken long-term potentiation, impairing memory formation and consolidation.
3.4.4 Reduced Neural Network Efficiency: Damage to myelin proteins in white matter and impaired axonal transport slow information transmission between brain regions and degrade synchronization.
3.4.5 Dysregulation of Autophagy and Apoptosis: Ultimately leads to selective neuronal loss, particularly in critical regions like the hippocampus and prefrontal cortex.
IV. Mechanism of Action and Effects of Non-Degenerative Brain Nutrition
Non-degenerative brain nutrition not only provides comprehensive, balanced nutrients essential for brain tissue growth and repair—including proteins, phospholipids, HA, ALA, EPA, etc.—but more importantly delivers their natural active structures. These structures possess special functions beyond mere nutrition.
4.1 Inhibition of Functional Brain Protein Degradation
Mechanism: The three-dimensional structure of non-denatured brain neurotrophic ultrafine powder resists degradation by gastric juices, enabling direct physical contact with the intestinal and intestinal lymphatic systems. This activates immature T cells to transform into specific Tregs (regulatory T cells). Tregs modulate effector T cells to prevent erroneous immune attacks on brain proteins, secrete anti-inflammatory factors to suppress chronic inflammation, and slow down the degradation of brain proteins.
4.2 Promoting Structural Formation and Abnormal Repair of Brain Proteins
Mechanism: Non-denatured brain neuro-ultrafine powder contains intact primary and secondary covalent cross-linking structures. Intestinal digestive enzymes selectively break down primary (peptide bond) covalent cross-links while preserving secondary covalent cross-links. This process yields small molecules such as cross-linked peptides and conjugated peptides, or serve as substrates for specific enzymes to enhance catalytic activity; or function as molecular chaperones to repair abnormal structures; or induce brain protein structure formation and abnormal structure repair through multiple pathways such as signal transduction.
4.3 Nutrient Supply
Mechanism: Small molecules like cross-linked peptides and conjugated peptides can further decompose into individual amino acids, directly participating in brain protein synthesis.
4.4 Effect
This dual-regulatory effect of inhibiting degradation and promoting synthesis yields significantly greater efficacy than the nutrient-supplying role of denatured proteins or peptides.
V. Tissue-Derived
5.1 Tissue-Derived Structure-Dependent Regulation
The primary functions of non-denatured nutrition (immunomodulation, activity regulation) are achieved through naturally active structures. These structures exhibit high specificity, exerting regulatory effects only on proteins with identical structures.
5.2 Corresponding Tissue Origin
Non-denatured brain nutrition utilizes fresh bovine or porcine brain tissue, including the cerebrum, midbrain, and cerebellum. Consequently, its protein structures are homologous to those found in the cerebrum, midbrain, and cerebellum.
VI. Suitable Populations
6.1 Individuals requiring brain growth and development: Infants, young children, and those needing brain and neural development support.
6.2 Individuals seeking brain anti-aging or addressing brain dysfunction.
Non-denatured Muscle Protein - Muscle Health
I. Overview
Non-denatured muscle protein ultrafine powder is a non-denatured functional food ingredient produced from fresh beef tenderloin or chicken breast. It is manufactured according to relevant standards using ultra-low-temperature ultrafine grinding (national patented technology) and ultra-low-temperature separation technology based on the air cabinet principle (proprietary technology).
II. Composition Ratios and Functions
2.1 General Classification

2.2 Protein Composition and Functions
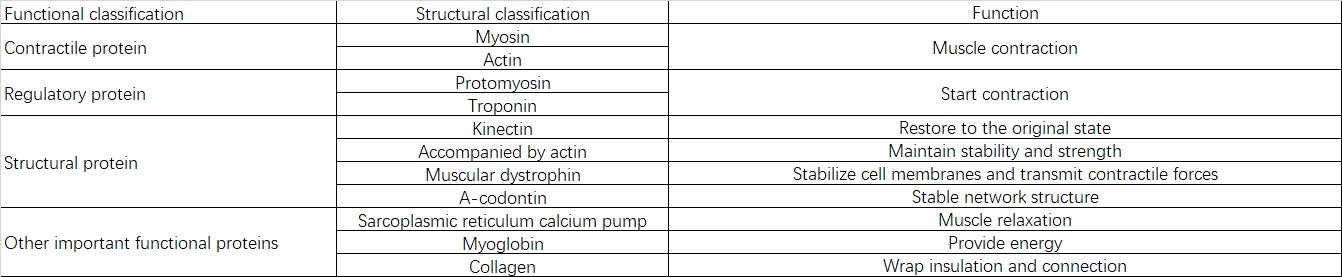
III. Causes of Sarcopenia
Sarcopenia centers on the progressive loss of skeletal muscle mass, strength, and function, resulting from multiple interrelated factors.
3.1 Imbalance Between Protein Synthesis and Degradation (Core Pathology)
3.1.1 Reduced Synthesis
Muscle protein synthesis rates decline with age. Even when stimulated (e.g., through nutrition/exercise), the synthetic response weakens, leading to synthetic resistance.
3.1.2 Increased Degradation
Overactivation of the ubiquitin-proteasome system and autophagy-lysosomal system, coupled with upregulation of genes such as Atrogin-1 and MuRF1.
3.2 Hormonal and Signaling Pathway Dysregulation
3.2.1 Decreased Anabolic Hormones
Significant impairment of the growth hormone/insulin-like growth factor-1 axis.
Decreased levels of sex hormones (testosterone/estrogen).
3.2.2 Inhibition of Anabolic Pathways:
Insulin resistance: Reduced efficiency of amino acid uptake and utilization in muscle.
Suppression of key anabolic pathways: e.g., diminished activation capacity of the mTOR signaling pathway.
Increased myostatin: Inhibits muscle growth.
Enhanced catabolic signals: Relative or absolute increase in glucocorticoids promotes protein breakdown.
3.3 Chronic Inflammation
Aging is accompanied by systemic, chronic, low-grade inflammation with elevated pro-inflammatory cytokines (e.g., IL-6, TNF-α).
IV. Mechanism of Action and Effects
Non-denatured muscle protein ultrafine powder not only provides comprehensive, balanced nutrients essential for muscle growth but also possesses “active structural” properties.
4.1 Degradation Inhibition Mechanism and Effects
Mechanism: Undenatured Muscle Protein Micropowder contains multiple undenatured muscle proteins with intact three-dimensional structures. These proteins resist degradation by gastric juices, reaching the small intestine intact. They are phagocytosed by M cells, enabling specific physical contact with the intestinal lymphatic system. activating immature T cells to transform into specific Tregs. This modulates effector T cells' erroneous attacks on various muscle proteins, secretes anti-inflammatory factors, and suppresses chronic inflammation.
Effect: Slows muscle protein degradation.
4.2 Mechanism and Effects of Promoting Synthesis
Mechanism: Non-denatured muscle protein ultrafine powder contains intact primary and high-order covalent cross-link structures. Intestinal digestive enzymes can specifically select and break down primary (peptide bond) covalent cross-linking structures while preserving higher-order covalent cross-links. This yields small molecules like cross-linked peptides and conjugated peptides, which then regulate protease catalytic activity in multiple ways—as substrates, molecular chaperones, or signaling molecules—to enhance muscle protein synthesis. Further degradation into individual amino acids enables direct participation in muscle protein synthesis.
Effect: Promotes muscle synthesis.
This dual-action regulation—inhibiting degradation while promoting synthesis—yields significantly greater efficacy than denatured proteins or peptides.
V. Tissue-Derived
5.1 Tissue-Derived Structure-Determined
The primary functions of non-denatured nutrition (immunomodulation, activity regulation) are achieved through naturally active structures. These structures exhibit high specificity, exerting regulatory effects only on proteins with identical structures.
5.2 Tissue-Specific Origin
Non-denatured muscle nutrition ingredients are sourced from beef tendon or chicken breast, both skeletal muscle tissues. Beef tendon exhibits 95% structural similarity, while chicken breast shows 85% structural similarity.
VI. Suitable Populations
6.1 Athletes: Individuals engaged in strength training and muscle-building.
6.2 Sarcopenia: Inhibits muscle degradation and promotes muscle synthesis.
Non-denatured spleen protein—Immune health
I. Overview
Non-denatured spleen protein ultrafine powder is a non-denatured functional food ingredient produced from fresh bovine spleen. It is manufactured using ultra-low-temperature ultrafine processing technology (national invention patent) and ultra-low-temperature separation technology based on the wind cabinet principle (proprietary technology), in accordance with relevant standards.
II. Component Ratios and Functions

2.1 Composition and Functions of Core Immune Response Protein Complexes
2.1.1 Immune-Related Proteins (Core Functional Complex): The most distinctive and functional fraction within spleen proteins.
Composition: Antibodies (immunoglobulins IgG, IgM, etc.) and their fragments.
Function: Core executors of humoral immunity.
2.1.2 Major Histocompatibility Complex Proteins
Composition: MHC Class I and MHC Class II molecules, widely expressed on antigen-presenting cell surfaces.
Function: Capture pathogen protein fragments (antigens) and “present” them to T lymphocytes.
2.1.3 Complement System Proteins
Composition: Multiple complement components including C3, C4, and complement activators.
Function: Form the “complement membrane attack complex” to directly perforate pathogen membranes; act as “chemokines” to attract immune cells to infection sites; mark pathogens to promote phagocytosis (complementary activity).
2.1.4 Cytokines and Chemokines
Composition: Interleukins, interferons, tumor necrosis factors, etc.
Function: Serve as “signaling molecules” between immune cells, precisely directing the initiation, enhancement, suppression, or termination of immune responses, and coordinating the behavior of various immune cell types.
2.1.5 Pattern Recognition Receptors
Composition: Toll-like receptors, etc.
Function: Recognize conserved molecular patterns of pathogens to initiate rapid innate immune responses.
2.2 Proteins Involved in Red Blood Cell Processing
2.2.1 Hemoglobin Degradation Enzymes
Composition: Heme oxygenase, biliverdin reductase, etc.
Function: Break down hemoglobin in aged red blood cells, releasing iron ions for reuse and converting heme into bilirubin.
2.2.2 Iron Storage and Transport Proteins
Composition: Ferritin is the most important representative.
Function: Safely and efficiently stores and buffers iron, preventing free iron from generating harmful free radicals, and releases iron as needed by the body.
2.3 Structural and Scaffolding Proteins
Composition: Extracellular matrix proteins (e.g., collagen, fibronectin) and cell adhesion molecules.
Function: Form the physical scaffold of the spleen's white pulp (periarterial lymphatic sheaths, lymphoid follicles) and red pulp; mediate lymphocyte homing, retention, and interactions between immune cells.
2.4 Metabolism and Antioxidant Enzymes
2.4.1 Antioxidant Enzymes
Composition: Superoxide dismutase, catalase, glutathione peroxidase.
Function: Scavenging reactive oxygen species (ROS) to protect splenic tissue from oxidative damage.
2.4.2 Nucleotide Metabolic Enzymes
Composition: Purine nucleoside phosphorylase, etc.
Function: Participates in purine metabolism within lymphocytes, critical for immune cell function.
2.5 Signal Transduction and Regulatory Proteins
Composition: Transcription factors including NF-κB, STAT family proteins, etc.
Function: Receive signals such as cytokines, translocate into the nucleus to regulate specific gene expression, and determine the activation, proliferation, or differentiation fate of immune cells.
III. Mechanism of Action and Effects
Non-denatured spleen protein ultrafine powder delivers intact, naturally conformation-preserved spleen proteins. Its core mechanism does not involve nutrient provision but rather functions as a complex, natural repository of bioactive molecules. It supplies regulatory signals and functional modules to the body—particularly the immune system—thereby exerting a “nutritional immunity” effect.
3.1 Local Intestinal Immune Effects
3.1.1 Passive Immune Effects
Non-denatured spleen protein ultrafine particles contain abundant immunoglobulins that reach the intestine intact under structural protection. Their antigen-binding fragments (Fab regions) maintain activity for a period, exerting local passive immunity. These Fab fragments may: - Directly neutralize specific viruses, bacteria, and their toxins, rendering them incapable of infection or toxicity; - Adhere to pathogens to block invasion; - Form complexes with pathogens or antigens that stimulate intestinal peristalsis and facilitate mucus encapsulation, accelerating their expulsion via feces and reducing their retention time and harm within the gut.
3.1.2 Maintaining Intestinal Barrier Function
Reducing Inflammatory Damage: By rapidly eliminating pathogens and toxins, it reduces direct attacks on intestinal epithelial cells and the resulting excessive inflammatory response, thereby protecting the integrity of the intestinal barrier.
Supporting Repair: Some studies suggest that other components in immunoglobulin preparations (such as growth factors) or the antibodies themselves may indirectly promote mucosal repair.
3.1.3 Modulating the Local Immune Environment
Antigen Buffering: Within the gut—the body's largest immune organ—excessive exogenous antigens (including food and pathogenic antigens) may trigger abnormal immune responses. Oral immunoglobulin binds and removes a portion of these antigens, reducing the burden on the intestinal immune system and the risk of misdirected attacks, thereby helping maintain immune tolerance balance.
Anti-inflammatory Effects: By reducing pathogen stimulation through the aforementioned mechanisms, it downregulates pro-inflammatory cytokine production, creating a local environment more conducive to tissue repair and microbial balance.
3.2 Systemic Immunomodulatory Effects
3.2.1 Induction of Peripheral Immune Tolerance as Antigens
The spleen contains immune-related proteins and antigens in their native, non-denatured forms. These substances may be recognized by the intestinal immune system as “friendly and familiar immune stimuli,” enhancing immune vigilance and responsiveness without triggering intense inflammation (oral tolerance pathway).
3.2.2 Direct Provision of Immunologically Active Components and Signaling Molecules
Releases small-molecule peptides with immunomodulatory activity within the gut. These peptide fragments function as signaling molecules recognized by gut immune cells (e.g., those within Peyer's patches), gently stimulating and training the immune system—akin to an “immune rehearsal.”
3.2.3 Interaction with the Immune Information Repository
Potential for inducing oral tolerance: For certain autoimmune predispositions, theories suggest that ingesting tissues containing self-similar antigens may aid in inducing immune tolerance.
IV. Tissue-Derived
4.1 Tissue-Derived Structure-Dependent
The primary functions of non-denatured nutrition (immunomodulation, activity regulation) are achieved through naturally active structures. These structures exhibit high specificity, exerting regulatory effects only on proteins with identical structures.
4.2 Corresponding Tissue Origin
The spleen, rich in diverse immune cells and factors, corresponds to immune tissue as its tissue origin.
V. Suitable Populations
5.1 Immunocompromised Individuals
5.2 Individuals with Digestive Disorders
Non-denatured Elastin—Vascular Health
I. Overview
Non-denatured elastin ultrafine powder is a non-denatured functional food ingredient produced from fresh bovine nuchal ligaments or bovine aortas. It is manufactured according to relevant standards using ultra-low-temperature ultramicronization (patented national invention technology) and ultra-low-temperature separation technology based on the wind cabinet principle (proprietary technology).
II. Component Ratios
2.1 Composition of Non-Denatured Elastin Ultrafine Powder (Bovine Nuchal Ligament) and Its Freeze-Dried Powder

2.2 Composition of Non-denatured Elastin Ultrafine Powder (Bovine Aorta) and Its Freeze-Dried Powder

III. Natural Active Structure
3.1 Primary Structure
The elastin amino acid sequence consists of alternating hydrophobic segments (rich in valine, alanine, glycine, and proline) and cross-linked segments (rich in lysine and alanine) arranged in tandem.
3.2 Higher Structure
The disordered amino acid sequence of elastin protein prevents the formation of regular secondary structures, lacking conventional II, III, or IV-level structures. Instead, it forms a three-dimensional disordered network polymer through covalent cross-links such as elastin cross-links.
IV. Mechanism of Action and Effects
Elastin is a non-nutritional protein whose primary function is not amino acid provision but rather its unique “natural active structure,” which exerts structural effects.
4.1 Peripheral Immune Regulation Mechanism and Effects
4.1.1 Mechanism: The multidimensional structure unique to non-denatured elastin activates the differentiation of Peyer's patch lymphoid T cells into specific regulatory T cells (Tregs). This modulates the immune system's erroneous attack on endogenous elastin and suppresses inflammatory responses.
4.1.2 Effects: Slows elastin degradation. With aging (beginning around ages 25-30), the body's ability to synthesize elastin declines sharply. Concurrently, due to immune dysregulation, existing elastin fibers become fractured, stiffened, and dysfunctional through glycation, oxidative stress (free radical damage), and enzymatic degradation (e.g., by matrix metalloproteinases). This is the primary cause of vascular hardening in the human body.
4.2 As a Cellular Signaling Molecule (Most Noted Function)
4.2.1 Mechanism: The surface of intact elastin molecules contains specific bioactive domains (e.g., cell-binding domains, chemotactic domains). When these domains maintain their native conformation, they can specifically bind to receptors (e.g., elastin-binding proteins) on the surfaces of fibroblasts, endothelial cells, and other cells. This binding signals cells to stimulate their own synthesis of additional collagen, elastin, and hyaluronic acid. This process induces endogenous regeneration, which is far more significant than merely supplying raw materials.
4.2.2 Effect: Contributes to fundamentally improving elasticity and firmness, restoring the elasticity of tissues such as blood vessels.
4.2 As a Substrate Modulating Enzyme Activity
4.2.1 Mechanism: Non-denatured elastin can be broken down in the gut into cross-linked peptides containing primary and secondary covalent cross-link structures. These cross-linked peptides serve as substrates for relevant structural modification enzymes, thereby upregulating their catalytic activity.
4.2.2 Effect: Promotes the formation or repair of elastin structures.
4.3 As a Structural Template and Scaffold
4.3.1 Mechanism: Non-denatured elastin, retaining large structural fragments and non-denatured cross-linked structures, may function as a “guiding scaffold” in vitro or in vivo. It directs newly synthesized elastin precursor molecules toward ordered arrangement and correct cross-linking, facilitating the formation of functional new elastic fiber networks.
4.3.2 Effect: Aids in repairing broken, disordered elastic fibers caused by photoaging or aging.
V. Tissue-Derived
5.1 Tissue-Derived Structure-Dependent
The functions of non-denatured proteins (immunomodulation, activity regulation) are structure-dependent. Their structure exhibits high specificity, regulating only proteins with identical structures.
5.2 Corresponding Tissue-Derived Sources
Tissues rich in elastin, such as blood vessels, skin, and alveoli.
VI. Suitable Candidates
6.1 Vascular Health: Hypertension, hyperlipidemia, atherosclerosis.
6.2 Skin Anti-Wrinkle: Skin laxity, wrinkles, loss of elasticity.
6.3 Pulmonary Insufficiency: Patients with emphysema, chronic obstructive pulmonary disease (COPD), etc.






