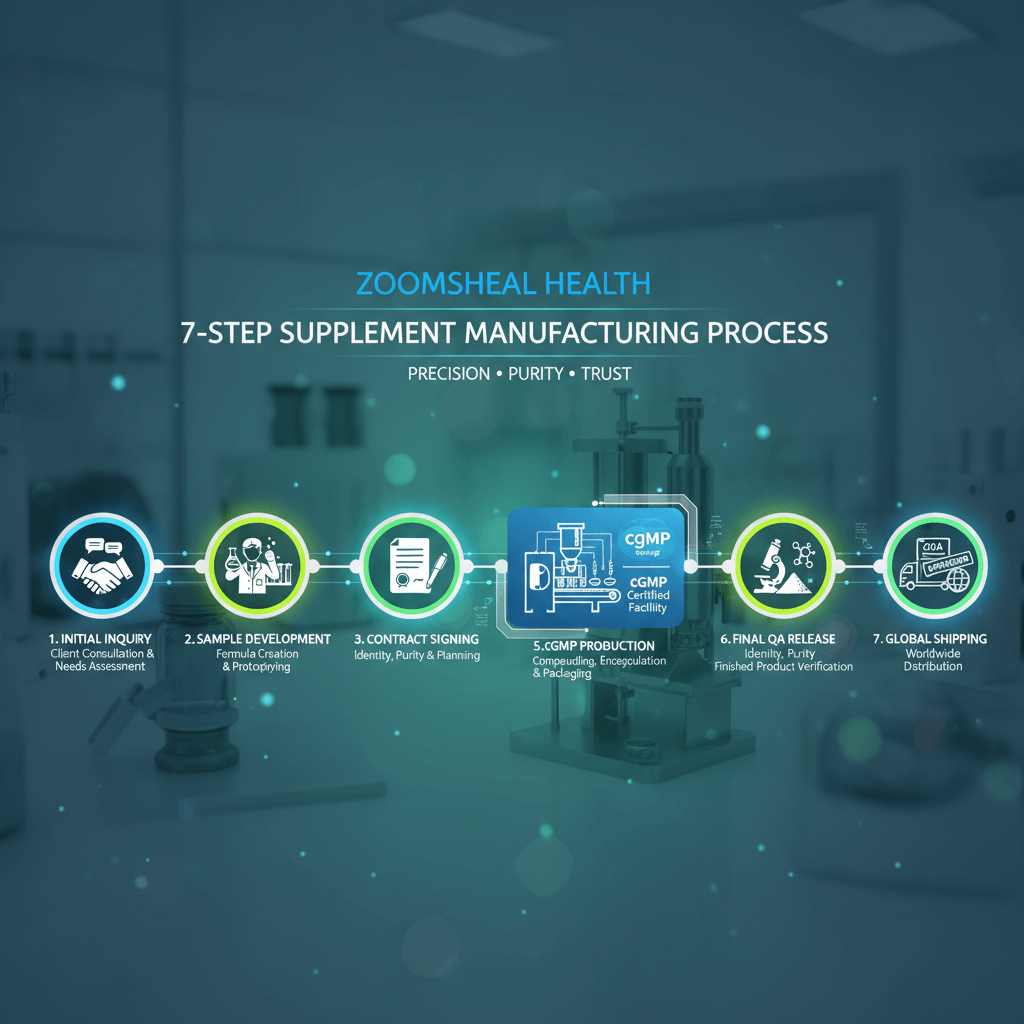
The Zoomsheal Health Advantage: A 7-Step Journey in Custom Supplement Manufacturing (OEM/ODM)
The dietary supplement landscape is defined by innovation, trust, and speed-to-market. For brand owners seeking to launch a unique, scientifically-backed product, choosing the right manufacturing partner is the single most critical decision. At Zoomsheal Health, we transform visionary concepts into compliant, high-efficacy supplements through a transparent and rigorous 7-step OEM/ODM process, ensuring quality from raw material sourcing to final logistics.
Our commitment to cGMP standards and specialized expertise in advanced formulations (from HPMC capsules to customized powders) makes us the ideal strategic partner for ambitious wellness brands globally.
Phase 1: Discovery and Conceptualization (The Partnership Begins)
The journey starts with the client's vision. Whether the client has a fully realized formula (OEM) or requires full concept development (ODM), our initial phase focuses on comprehensive understanding and strategic alignment.
Step 1: Inquiry, Contact, and Project Scoping
-
Initial Engagement: A brand contacts Zoomsheal Health via our dedicated channels (phone, email, or online portal) to discuss their project. This is the stage for sharing the fundamental concept: target demographic, desired health benefit (e.g., gut health, bioenergetics), preferred delivery form (capsule, softgel, powder), and estimated order volume (MOQ).
-
Scientific and Market Alignment: Our R&D team reviews the concept against current market trends (e.g., fermented ingredients, adaptogens) and regulatory feasibility. We assess the proposed ingredients for known stability conflicts and potential synergies, offering initial scientific recommendations to maximize product differentiation and consumer appeal.
-
Quotation and Timeline Drafting: Based on the initial complexity and volume, a preliminary non-binding quote and a project timeline are drafted, clearly outlining the subsequent stages, including sample development fees.
Phase 2: Scientific Validation and Commercial Agreement
This phase moves the project from concept to a validated, manufacturable product, ensuring both scientific efficacy and commercial feasibility.
Step 2: Custom Formulation and Sample Development
-
Proprietary Formulation: For ODM projects, our in-house chemists develop a proprietary Bill of Materials (BOM), selecting suppliers for the highest-grade raw materials (e.g., standardized herbal extracts, Non-GMO powders). A key focus is on ensuring bioavailability—we may utilize technologies like micro-encapsulation or the inclusion of absorption enhancers (e.g., Piperine) to optimize nutrient uptake.
-
Bench Samples and Stability Testing: A small bench batch is produced for formulation refinement. This sample is crucial for two reasons:
-
Client Approval: The sample is shipped to the brand owner for sensory (taste, texture, appearance) and formula approval.
-
Pre-Stability Analysis: We conduct initial stress testing (e.g., high heat/humidity exposure) on the sample to predict the product’s long-term behavior and select the optimal container-closure system.
-
-
Regulatory Review: The final formula and label claims are reviewed to ensure full compliance with target market regulations (FDA, EFSA, etc.).
Step 3: Contract Finalization and Intellectual Property (IP) Agreement
-
Contract Drafting: Once the sample is approved and all specifications (formula, packaging, quantity, pricing) are locked, a formal Manufacturing Agreement is drafted. This document defines key terms, including MOQs, quality assurance metrics, payment schedules, and lead times.
-
IP Protection: A critical component is the Non-Disclosure Agreement (NDA) and IP clause. For OEM (client-owned formula), Zoomsheal Health guarantees the protection and confidentiality of the client's proprietary formula. For ODM, IP ownership of the final formula is clearly defined, often residing with the client upon contract signing.
-
Purchase Order (PO) and Deposit: Upon contract execution, the client issues a formal PO and a required deposit to initiate the raw material procurement and production scheduling.
Phase 3: Production, Quality Control, and Logistics
With the contract secured, Zoomsheal Health mobilizes its cGMP-certified facilities for the high-volume manufacturing run.
Step 4: Raw Material Procurement and QC Testing
-
Approved Supplier Vetting: We procure all components, from active ingredients to excipients and packaging materials, exclusively from Zoomsheal Health’s pre-vetted and cGMP-compliant supplier list.
-
Incoming Material Quarantine and Testing: Upon arrival, all raw materials are immediately quarantined. Our in-house QC laboratory conducts comprehensive tests, including:
-
Identity Testing: Verifying the material is what it claims to be (FTIR, HPLC).
-
Purity Testing: Screening for microbial contamination, heavy metals (Lead, Arsenic), and solvent residues. Only materials passing all QC checks are released for production.
-
Step 5: Full-Scale cGMP Manufacturing and In-Process Control
-
Blending and Mixing: Materials are precisely weighed according to the BOM and mixed in validated high-shear blenders to ensure homogeneity (uniform content) across the entire batch. In-process QC checks are performed to verify blend uniformity.
-
Dosing and Encapsulation/Tableting: The blend moves to the designated production line (e.g., high-speed capsule fillers, tablet presses). Advanced equipment ensures precise dosing consistency. For capsules, we verify fill weight and sealing integrity. For tablets, we monitor hardness and dissolution rates.
-
In-Process QC: Throughout production, samples are pulled for quality checks on weight variation, disintegration time, and visual compliance.
Step 6: Final Product Testing and Quality Release
-
Finished Product QC: The final, packaged supplement batch undergoes the most stringent battery of tests to confirm regulatory and label compliance.
-
Potency Testing: HPLC or other validated methods confirm that the active ingredients meet the label claim from the first unit to the last.
-
Microbial and Contaminant Testing: The finished product is re-tested for pathogens and contaminants.
-
Formal Stability Study Initiation: Long-term shelf-life studies (accelerated and real-time) are formally initiated at this stage to justify the product’s expiration date.
-
-
Quality Assurance (QA) Release: Once all QC tests are successfully completed, the QA department issues the Certificate of Analysis (COA) and Certificate of Manufacture (COM), officially releasing the finished goods for packaging and shipping.
Step 7: Packaging, Labeling, and Global Logistics
-
Final Packaging and Serialization: Supplements are filled into the client's approved packaging (bottles, blister packs, pouches). Automated systems apply labels and safety seals, ensuring accurate lot number and expiration date printing (serialization).
-
Regulatory Compliance Check: The final packaged unit is checked against the approved label file to ensure mandatory elements (Supplement Facts panel, allergen warnings, disclaimers) are correctly positioned.
-
Shipping and Distribution: The client's shipment is prepared and palletized. Zoomsheal Health manages the final logistics, coordinating with trusted global carriers and providing necessary export documentation to ensure safe, timely delivery to the client’s warehouse or chosen distribution center.
Zoomsheal Health ensures that every step of this 7-step process is documented, compliant, and focused on delivering a premium, market-ready product that truly represents the client's brand ethos.